TA・RA
名前と所属

Tanawat Kanjanaboonmalert Research Field: Solid oxide fuel cell
Research Period: 2006-2009
Research Title: Electrode Activity of a Solid Oxide Fuel Cell anode
consisting of nickel alloy, cerium oxide and
titanium oxide for the direct oxidation of methane.
Affiliation: Nagaoka University of Technology
研究内容
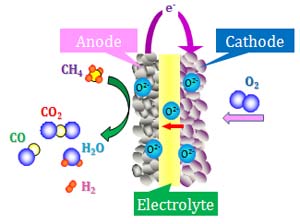
Solid Oxide Fuel Cells
Solid oxide fuel cells (SOFCs), all-ceramic device invented for conversion of chemical fuels directly into electrical power, have attracted much attention because of their distinguished advantages over other types of fuel cells such as high electric efficiency, high-quality exhaust heat and system compactness.
Fig. 1 The schematic model of a solid oxide fuel cell.
This work has been focused on the development of an anode material for the SOFCs using methane (CH4) as a fuel. Direct oxidation of methane results in an increase in the efficiency of electric power generation.Foreign metal ion doped-CeO2 offers the advantage of promoting the charge-transfer reactions over the whole area of the electrode-gas interface. A mixed oxide (CeO2-TiO2) was synthesized by a sol-gel method and characterized by XRD, SEM, and BET.
The conductivity of CeO2-TiO2 was studied by the dc 4-probe technique. The electrochemical measurement was conducted by a single cell composing of the Ni-based cermet anode, samarium-doped ceria (SDC) electrolyte (dia. 0.3 mm) and Sm0.5Sr0.5CoO3-SDC (SSC-SDC) cathode.
Results
 |
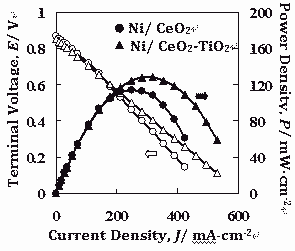 |
It was found that the doping of Ti4+ ions into CeO2 produces a porous network microstructure resulting in an increase of the electrical conductivity. The substitutional incorporation of Ti4+ ions into the CeO2 lattice could ease the valence change of Ce4+ to Ce3+, which contributes to a good mixed ionic and electronic conduction of the cermet anode. This mixed oxide can retard the agglomeration of Ni particle deactivating the cell performance. The maximum power density at 750°C in 10vol% CH4 diluted in Ar was 130 mW·cm-2 for the cell using the Ni/CeO2-TiO2 (20 mol% Ti) anode, which was higher than that, 110 mW·cm-2, for the cell using the Ni/CeO2, as shown in Fig. 2.
This enhancement is caused by a higher of electrical conductivity of CeO2-TiO2 mixed oxide system. Moreover, the ratio of H2/CO from the cell using Ni/CeO2-TiO2 anode was higher than that of Ni/CeO2 anode, which shows an effect of the mixed oxide on the electrochemical oxidation of methane.
Fig. 2 Cell performance among different anode cermets at 750°C under 10vol% CH4 diluted with Ar.
Present Work
The gas products from the anode are analyzed as a function of the current density in order to understand the reaction mechanism in relation to an enhancement of the cell performance.
